درمان گیاهی دل درد
کپسول نرم لومکس باریج
- درمان اسهال غیر عفونی
- کاهش دلپیچه و کاهش دفعات دفع











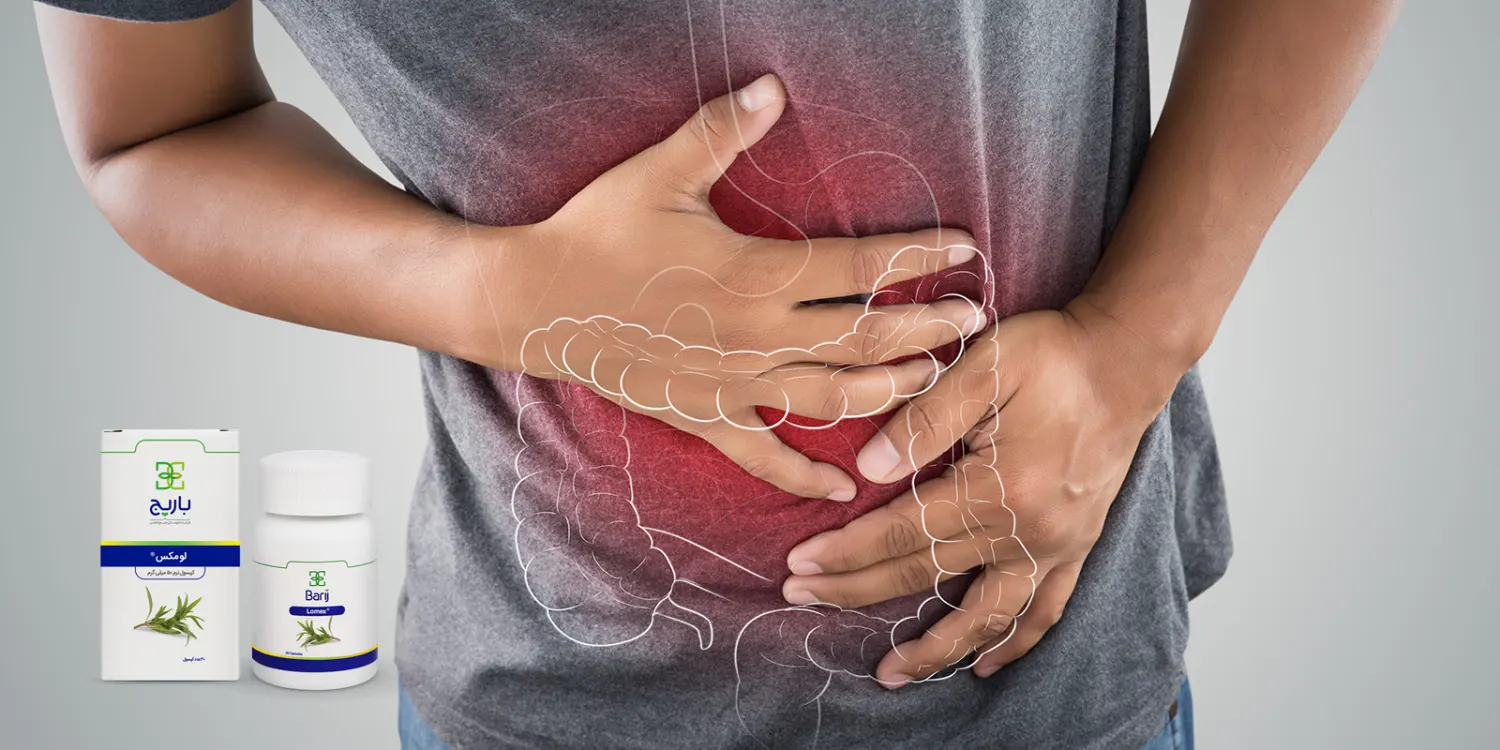
درمان اسهال غیرعفونی
تشکیل شده از اسانس مرزه
درمان گیاهی دل درد











کپسول نرم ۵۰ میلیگرم، در ظرف دارو همراه با بروشور درجعبه مقوایی
استاندارد شده بر اساس وجود ۲۲/۵ تا ۱۷/۵ میلیگرم کارواکرول در هر کپسول نرم
اسانس مرزه (Saturejahortensis)
در دوران بارداری و شیردهی مصرف نشود.
در طول مدت درمان؛ مصرف فراوان مایعات از جمله ORS ضروریست.
در صورت تداوم اسهال بعد از ۳ روز درمان، وجود تب یا مشاهده خون در اسهال؛ با پزشک مشورت شود.
هر ۸ ساعت یک عدد کپسول نرم، به مدت ۳ روز
(کلیه داروها با تجویز پزشک باید مصرف شوند)
کپسول نرم لومکس باریج برای کدام بیماریها تجویز میشود؟
کپسول لومکس باریج برای درمان اسهال غیرعفونی، کاهش دل پیچه، کاهش تعداد دفعات دفع و مدت اسهال تجویز میشود.
برای درمان اسهال چه دارویی توصیه میشود؟
برای درمان اسهال غیرعفونی کپسول نرم و قطره لومکس باریج توصیه میشود.
دستور مصرف کپسول لومکس باریج چیست؟
هر 8 ساعت یک کپسول نرم به مدت 3 روز باید مصرف شود.
منع مصرف کپسول لومکس باریج چیست؟
در دوران شیردهی و بارداری این دارو نباید مصرف شود.
عوارض جانبی مصرف کپسول لوکس باریج چیست؟
کپسول نرم لومکس باریج از گیاه مرزه تولید میشود و این گیاه هیچ عوارض جانبیای ندارد.
داروها و مکمل های گیاهی عالی با مواد با کیفیت ساخته می شوند. در باریج اسانس، ما خلوص گیاهان، کنترل در روش های تأمین و کیفیت بی نظیر را تأیید می کنیم.
ما اعتقاد داریم که شما لیاقت این را دارید که دقیقاً بدانید در داروها و مکمل های شما چیست و چه مواردی غیر از گیاهان وجود دارد. در باریج، هر عنصر و محصول نهایی برای اطمینان از خلوص، کیفیت و قدرت در آزمایشگاه پیشرفته ما آزمایش می شود.

 روغن گل محمدی باریج (موضعی)
روغن گل محمدی باریج (موضعی)
سحر –
من استفاده کردم و. راضی بودم
باریج اسانس –
سپاس دوست عزیز